Abstract

Introduction: Immune tolerance induction (ITI) has long been the standard of care for treatment of patients with hemophilia A complicated by an inhibitor. Although the approval of emicizumab to treat hemophilia has led to new debate as to the role of ITI, many continue to advocate for its use to restore the effectiveness of factor VIII for treatment of bleeding events and major surgery. Unfortunately, ITI is cumbersome to undertake, and providers may perceive that some families are unable to complete the complex treatment. This opens the door for bias and health inequities. To better understand potential health inequities in hemophilia care, we sought to explore the association of race and ethnicity, as well as patient and facility characteristics, on the receipt of ITI among patients with hemophilia A in the United States (US).
Methods: This cross-sectional study used data from the Community Counts (CC) Registry, which is a collaboration of the American Thrombosis & Hemostasis Network (ATHN), Centers for Disease Control and Prevention (CDC), and the Hemophilia Center Network (HTCN) using the ATHN study manager. The Registry collects data from patients that received care from more than 135 hemophilia treatment centers (HTC) across the US. Included in this analysis were patients with severe hemophilia A who entered the CC Registry between 2013-2017, were ≥5 years of age at the study entry, and had a history of an inhibitor (n=614). Data from 2013-2017 were selected to represent the pre-emicizumab era. The primary outcome was ever receiving ITI treatment, which was ascertained in the initial CC Registry visit form. The proportion receiving ITI was examined according to race/ethnicity, the age and year that an inhibitor was detected, age at study entry, current health insurance, pre-ITI peak inhibitor titer, and HTC size, dichotomized into having <40 or ≥40 patients with severe hemophilia A in the CC registry. Unadjusted prevalence ratios (PR) and corresponding 95% exact confidence intervals (CI) were computed with contingency tables.
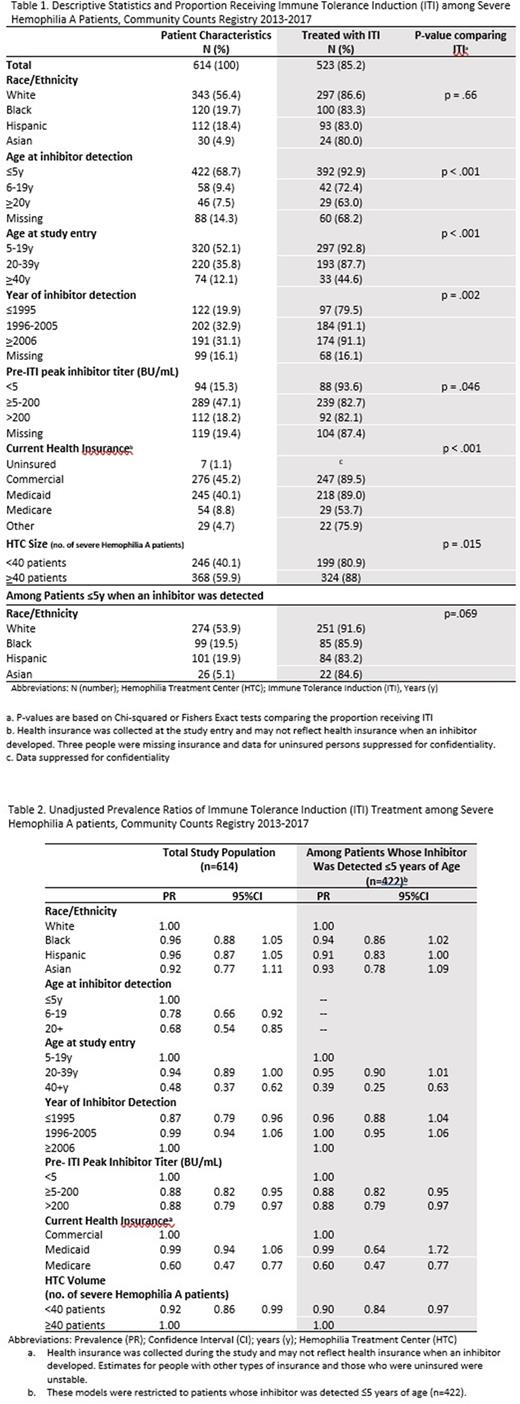
Results: Among 614 patients included in the study, slightly over half (56.4%) were White, 19.7% were Black, 18.4% were Hispanic, and 4.9% were Asian (Table 1). The average age at the study entry was 22.2 [standard deviation (SD =14.3)] years and the average age at inhibitor detection was 5.6 (SD=9.9) years. Most patients had a high titer inhibitor (≥5 BU/mL).
Approximately 85.2% of patients in the study received ITI; results were not statistically different by race-ethnicity. However, in an analysis restricted to patients whose inhibitors were detected ≤5 years of age, Black (PR=0.94, 95%CI 0.86, 1.02) and Hispanic (PR=0.91, 95%CI 0.83, 1.00) patients were non-significantly less likely to receive ITI compared to White patients (Table 2).
Receipt of ITI was also significantly lower among people currently receiving care from HTCs with <40 vs ≥40 patients (PR=0.92, 95%CI 0.86,0.99). Patients who were older when their inhibitor was detected (ages 5-19 years and ≥20 years vs. ≤5 years), were older during the study (ages ≥40 years vs. 5-19 years), had their inhibitor detected in 1995 or earlier vs 2006 or later, or had Medicare insurance vs commercial insurance were less likely to receive ITI. Additionally, those who had an inhibitor titer ≥5 BU/ml vs < 5 BU/ml were less likely to be treated with ITI (Table 2).
Conclusion: Most patients with severe hemophilia A with history of an inhibitor were treated with ITI. However, larger HTCs were more likely to implement ITI and those receiving Medicare were less likely to receive ITI. Additionally, among patients whose inhibitors were detected at ≤5 years of age, Black and Hispanic patients with severe hemophilia A had non-significantly lower rates of ITI for the treatment of inhibitors compared to White patients. Although the role of ITI may evolve with growing use of emicizumab and the introduction of new products, understanding characteristics that influence care will be relevant going forward and potentially applicable to other complex therapies such as gene therapy.
Disclosures
Kempton:Grifols: Honoraria; Novo Nordisk: Consultancy, Research Funding; Genentech: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.